We use the most common isotopes. Molecular mass ofCO 2 Molecular mass of carbon 2 Molecular mass of oxygen 2 atoms of oxygen are present Molecular mass of carbon 12.

How To Calculate A Molecular Formula Molecular Chemistry Class Molar Mass
For simple molecules a condensed or semi.

. Furthermore you also need to know the molar mass of the solute. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. How to calculate Molecular Weight.
Next divide the molecular weight by the empirical formula weights to come up with a ratio. How to find moles in the solution is to calculate how many molecules the solution contains. The manual way of finding molar mass using a molecular weight formula is as.
Calculate molecular mass by. 15999400 Mono-isotopic molecular masses. Where l i is the length of the side i and the sum runs over all of the sides in contact with the waterThis formula is quite straightforward to use in principle but in practice actually finding the information you need isnt easy.
There are 2 ways used for finding molar mass and calculating molecular weight. For example use the formula CH4 for methane which comprises 1 atom of carbon and 4 atoms of hydrogen. To find a molecular formula start by calculating the number of moles and the molecular weight of the gas using their respective formulas.
However except for very simple substances molecular chemical formulae lack needed structural information and are ambiguous. Enter the molecular formula of the molecule. Molecular mass of CO 2 12 16 X 2 12 32 44 g.
Also to do this you need to know the volume of the solution and how many solutes has been dissolved in the solution. It gives the smallest whole number ratio of elements in a compound using subscripts following element symbols. Mass of 1 mole 6023 X 1023 molecules of CO2 is about 44g.
The empirical formula is also known as the simplest formula in chemistry. If youre actually in the location of the water and the surfaces in contact with it the simplest way to find the wetted perimeter is to physically. The percentage by weight of any atom or group of atoms in a.
The formula mass formula weight of glucose is 30 either no units or else grams per mole while the molecular mass molecular weight is 180156 gmol. This is how to calculate molar mass average molecular weight which is based on isotropically weighted. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance.
In a copy of the Periodic Table of Elements find the relative atomic mass of each element in the molecule. The atomic weights used on this site come from NIST the National Institute of Standards and Technology. C6H12O6 PO4H2CH212CH3 Standard Masses Standard Formula Weight.
To calculate molecular weight start by listing all elements present in the molecule. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. We need to find the molecular mass of carbon dioxide.
Molecular Mass for H2O 18015286 Da. A molecular formula enumerates the number of atoms to reflect those in the molecule so that the molecular formula for glucose is C 6 H 12 O 6 rather than the glucose empirical formula which is CH 2 O. In some cases the empirical formula is the same as the molecular formula which gives the actual number of atoms in a compound eg H 2 O.
You can try and take a small part of your original sample figure out how many bacteria there are in that part and then calculate to approximate out how many there might have been in the original. The first way includes all the manual calculations and the other way includes finding molar mass and molecular weight using a molecular formula calculator. The empirical formula is often the same as the molecular formula but not always.
Then add together the atomic weight of each of the atoms in the formula which you can find using the periodic table. The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 112 of the mass of a neutral carbon-12 12 C. The formula mass and molecular mass of water H 2 O are one and the same while the formula and molecular mass of glucose are different from each other.
For example the molecule acetylene has molecular formula C 2 H 2 but the simplest integer ratio of elements is CH. Then count the atoms using the molecular formula. Molecular mass of oxygen 16.
In the same way that you can take a poll of 1000 people and apply it to 10000 people to figure out how they might think about a certain subject.
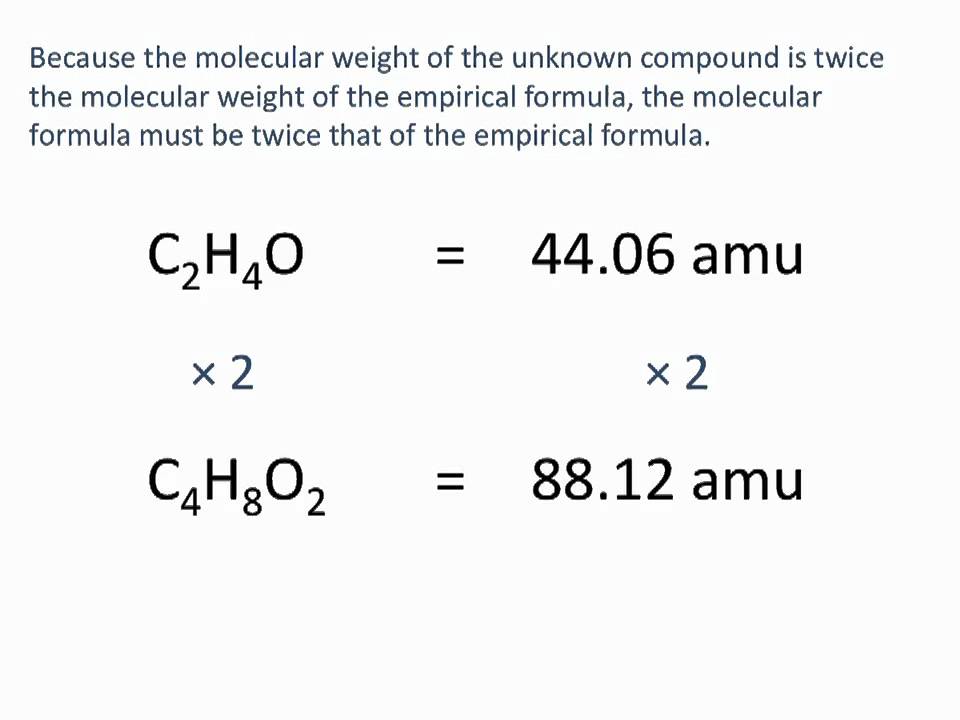
Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular

Calculating Molar Solubility Molarity Solubility High School Chemistry Chemistry Teacher

How To Calculate A Molecular Formula Molecular Chemistry Class Molar Mass

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular
0 Comments